Which of the Following Would Form an Electrolyte Solution
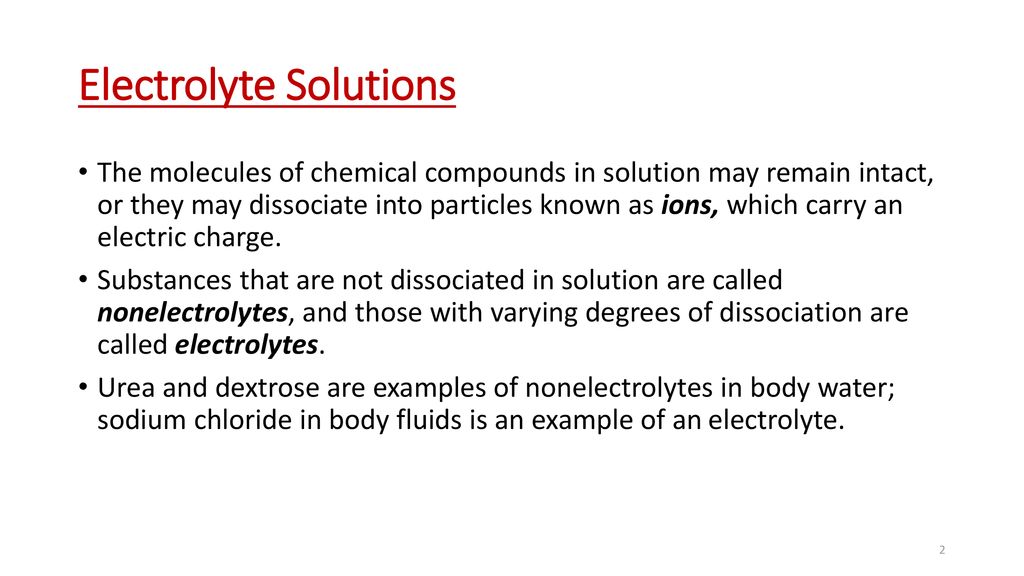
Sodium concentration affects serum osmolality and extracellular fluid volume. Substances that dissolve partially or dissociate partially in water.

To Identify Given Solutions As Electrolyte And Non Electrolyte Neb Class 9 Electrolytes Covalent Bonding Solutions
Chloride helps maintain osmotic pressure.
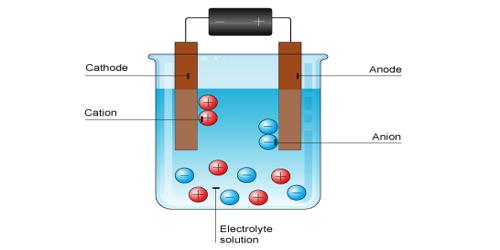
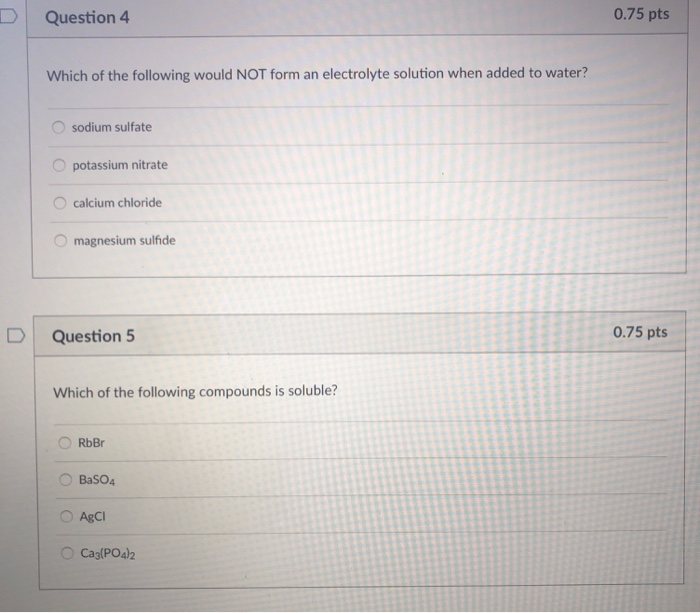
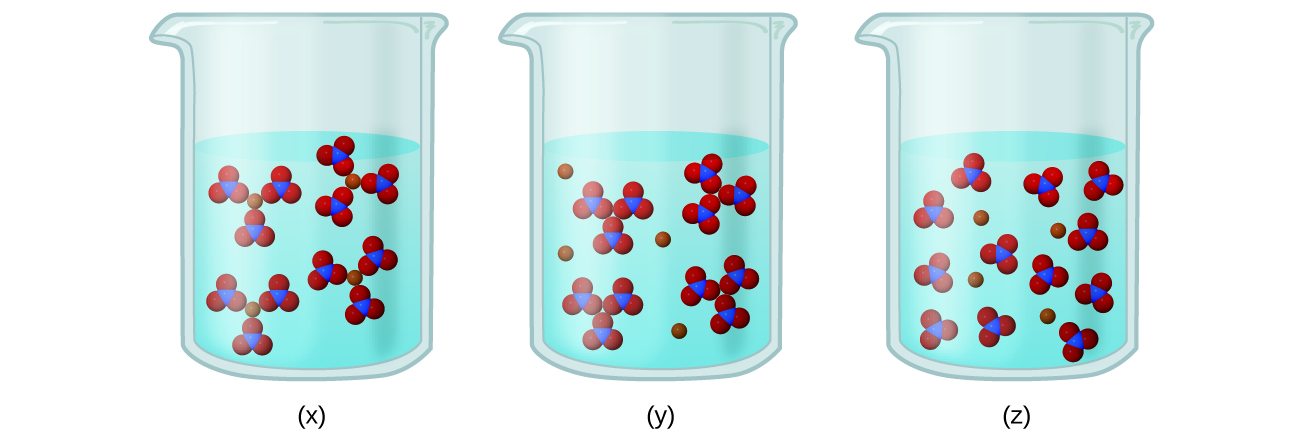
. DI Question 4 075 pts Which of the following would NOT form an electrolyte solution when added to water. It will definitely squander the time. Electrolyte is a substance that dissociates into ions in a solution and acquires the capacity to conduct electricityElectrolysis is the dissociation of an electrolyte into ions at the electrodes by the passage of electric current.
If electrolyte strong or weak. Strong electrolyte strong acid Identify NaCl. Sodium and chloride the major electrolytes in extracellular fluid exert most of their influence outside the cell.
Please check my answers 1. O RbBr O BaSO4 AgCl. However below in the same way as you visit this web page it will be for that reason no question easy to acquire as well as download guide which of the following would form an electrolyte solution.
In the human body electrolytes have. Salt in water is sodium. Oil in water B.
Which of the following would form an electrolyte solution. Let citizens speak freely and. Build and maintain public roads B.
Thus the correct option is B. Protect citizens from attack C. What precipitate is most likely formed from a solution containing Ba2 Na1 OH-1 and CO3-2.
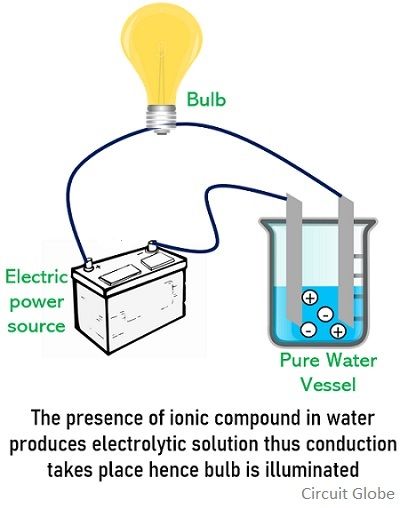
The substances that can dissociate into ions in water can act as electrolytes. When humans sweat we lose ions necessary for vital bodily functions. Lime water is.
Weak electrolyte weak acid Identify sugar. Interchange Book 1 Unit 4 Simple Present u0026 Would Verb for Invitations. Which of the following would form an electrolyte solution when added to water.
Whereas a covalent compound does not dissociate into ions. O sodium sulfate O potassium nitrate calcium chloride O magnesium sulfide D Question 5 075 pts Which of the following compounds is soluble. Major Electrolytes Outside the Cell.
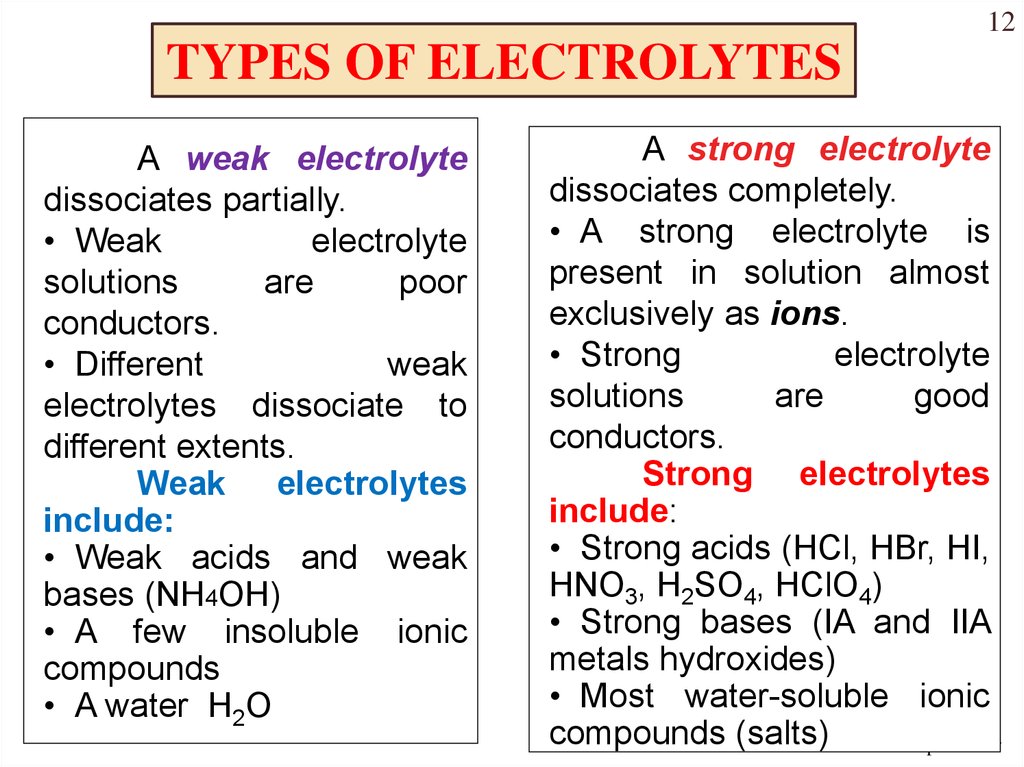
Which of the following is a non-electrolyte. 100 dissociation All water soluble ionic compounds strong acids and strong bases Weak Electrolytes. To replenish them we need to consume more ions often in the form of an electrolyte solution.
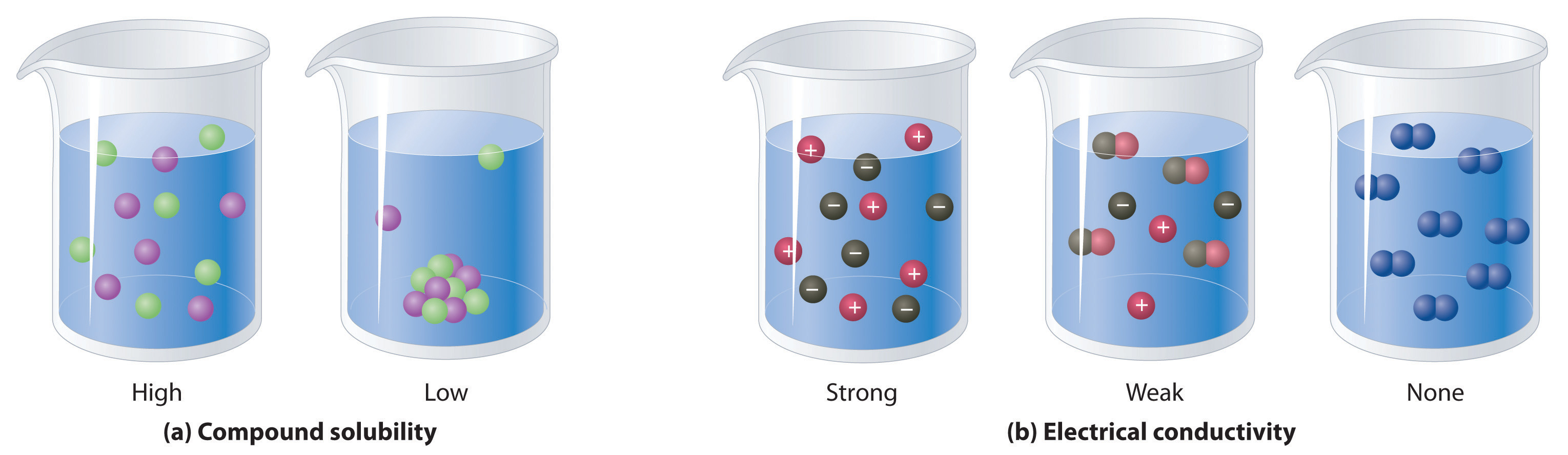
Sodium also helps nerve and muscle cells interact. If a high proportion of the solute dissociates to form free ions the electrolyte is strong. Among the following the solutions that are electrolytes are dil.
Group of answer choices. In the midst of guides you could enjoy now is which of the following would form an electrolyte solution below. Therefore we can conclude that form a non-electrolyte solution in water.
Which of the following would form an electrolyte solution. A solute that does not form ions or conduct electricity in solution. Group of answer choices.
Use these video links to help. It is your entirely own grow old to be in reviewing habit. Thus it does not act as an electrolyte.
As understood achievement does not suggest that you have astounding points. Which kind of policy was the troubled asset relief program TARP. Gatorade as an electrolyte solution The sports drink Gatorade advertises that it contains electrolytes because it contains sodium potassium magnesium and other ions.
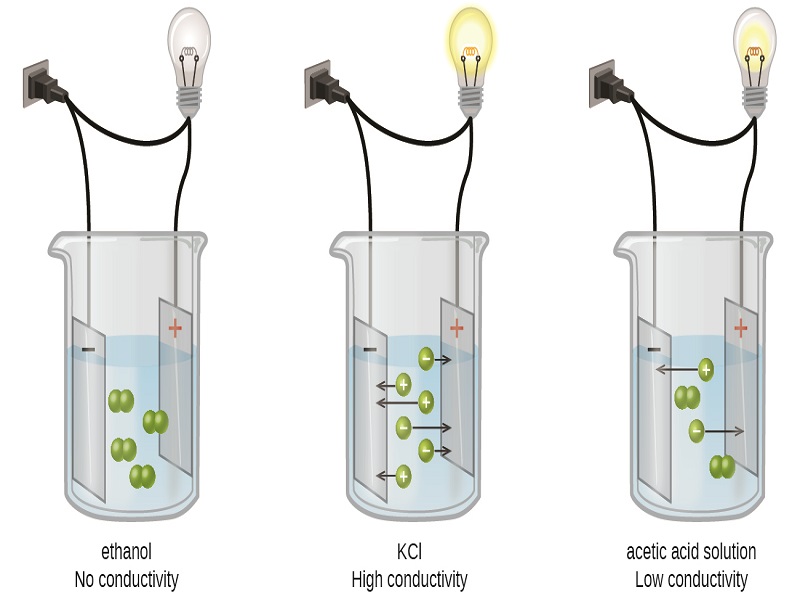
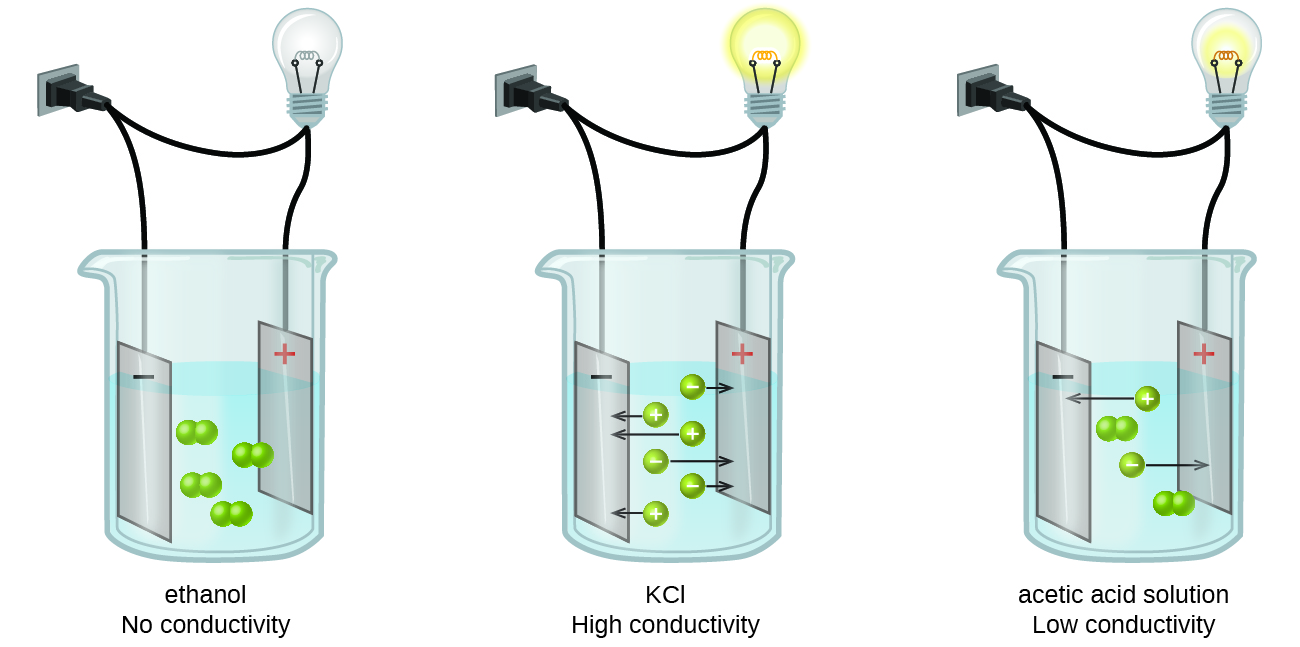
Substance that dissolved in water produces a solution that conducts electricity. Dilute HCl dissociates into and ions. If most of the solute does not dissociate the electrolyte is.
What would form an electrolyte solution. Sand in water C. This is just one of the solutions for you to be successful.
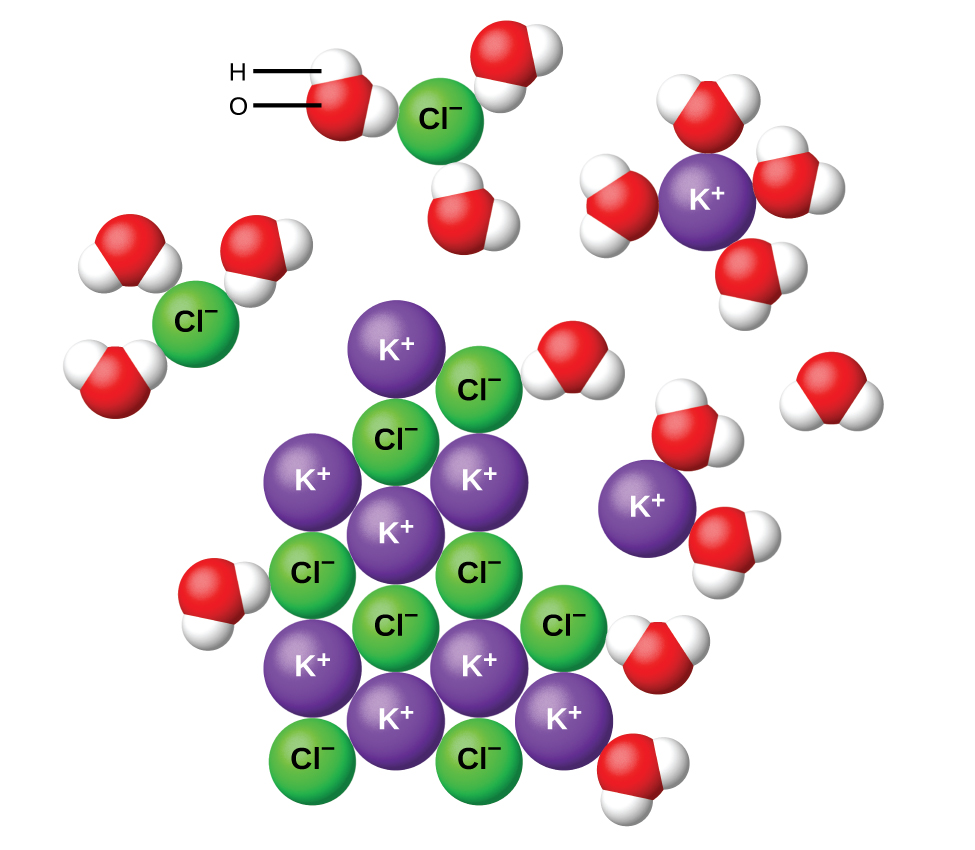
During the electrolysis of silver nitrate solution silver gets deposited at the cathode while the anode loses an equal amount of Ag. Substances that dissolve completely in water. Therefore this type of solution is known as an electrolyte.
Electrolyte Activity Fill out the chart for each of the following substances to determine if it would form an electrolyte solution in water. Which of the following would form an electrolyte solution when added to water. The Following Would Form An Electrolyte Solution Yeah reviewing a ebook which of the following would form an electrolyte solution could add your near links listings.
Ringers lactate solution sodium lactate solution and Hartmanns solution is a. HCI and Lime water. Hence it is not able to conduct electricity.
Chemistry questions and answers. It conducts electricity due to the presence of ions in the aqueous phase. Operate public schools D.
Sugar in water D. Ionic AcidBase or Covalent. An electrolyte in a solution may be described as concentrated if it has a high concentration of ions or dilute if it has a low concentration.
The statement which of the following would form an electrolyte solution that you are looking for. Following Would Form An Electrolyte Solutiongovernment be more likely to do than an unlimited government.
What Are Some Examples Of A Strong Electrolyte Quora

Mechanism Of Electrolytic Conduction Qs Study

Nonelectrolytes Vs Electrolytes Lists Solutions Compounds Video Lesson Transcript Study Com
Electrolyte And Non Electrolyte Docx
4 1 General Properties Of Aqueous Solutions Chemistry Libretexts
Difference Between Electrolytes And Nonelectrolytes With Comparison Chart Circuit Globe
Aqueous Solutions Of Electrolytes Prezentaciya Onlajn
What Is The Difference Between Electrolytes And Non Electrolytes Quora
Electrolyte Solutions Milliequivalents Millimoles And Milliosmoles Ppt Download

Electrolytes And Nonelectrolytes Chemistry For Non Majors

Electrolyte And Nonelectrolyte Solutions Introduction To Chemistry

Electrolytic Cells Video Lesson Transcript Study Com

Electrolytes Definition Overview Expii
Nonelectrolyte Definition And Examples In Daily Life Studiousguy
Solved Di Question 4 0 75 Pts Which Of The Following Would Chegg Com

Comments
Post a Comment